Aveed is the U.S. equivalent to the long-acting testosterone formulation known in other parts of the world as Nebido, and it was recently approved by the FDA. Aveed is being touted as having “major advantages” over T. cypionate and T.enanthate, including fewer injections (about every 2 to 3 months), less fluctuation in testosterone levels, and possibly less risk of erythrocytosis.
The US Food and Drug Administration (FDA) has approved testosterone undecanoate injectable (Aveed, Endo Pharmaceuticals), for the treatment of men with hypogonadism, but with a boxed warning and very strong prescribing restrictions.
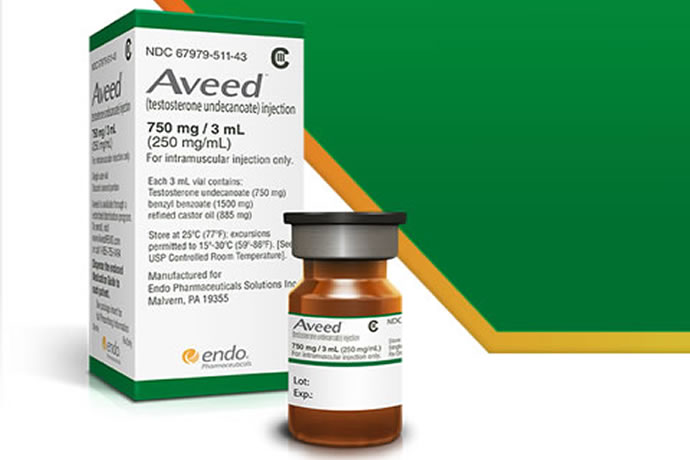
Aveed is a long-acting depot formulation of testosterone in castor oil and benzyl benzoate. It offers a novel dosing schedule, with a single 3-mL (750 mg) intramuscular injection given once at initiation of therapy, at 4 weeks, and then every 10 weeks thereafter.
The approval follows 3 previous rejections of Aveed by the FDA for safety and risk/benefit concerns and comes just a month after the FDA announced that it is investigating cardiovascular safety data for all testosterone preparations.
The FDA is requiring that Aveed’s label contain a boxed warning regarding the risks of serious pulmonary-oil microembolism (POME) and anaphylaxis and is making the product available only through a restricted distribution scheme known as a risk evaluation and mitigation strategy (REMS) to ensure that it is used only in men for whom the benefits outweigh the risks.
REMS Revised; Aveed Advantages Over Other Products
The REMS requires that patients must be observed for 30 minutes following injection to rule out serious POME or anaphylaxis, that healthcare settings and providers be specially certified in order to prescribe and dispense Aveed, and that they must have on-site equipment and trained personnel to manage such emergencies.
Aveed Drug Description
Aveed (testosterone undecanoate) injection contains testosterone undecanoate (17ß-undecanoyloxy-4-androsten-3-one) which is an ester of the androgen, testosterone. Testosterone is formed by cleavage of the ester side chain of testosterone undecanoate.
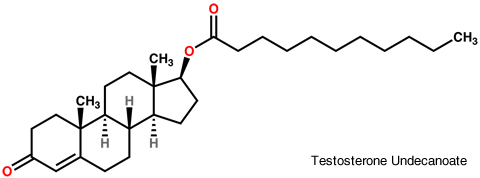
Aveed is a clear, yellowish, sterile oily solution containing testosterone undecanoate, a testosterone ester, for intramuscular injection. Each single use vial contains 3 mL of 250 mg/mL testosterone undecanoate solution in a mixture of 1500 mg of benzyl benzoate and 885 mg of refined castor oil. See the Aveed monograph.
Aveed is considered “specialty medication” which means:
- It is very expensive. A typical fill can cost $882 or more for 1 vial (3ml) of Aveed 750mg/3ml.
- Patients in need of this drug will usually find most of the cost paid by an insurance company, government or non profit organization.
- Most retail pharmacies will not stock this medication.
Aveed is the same product known as Nebido which is available in more than 50 countries across Europe, Asia Pacific and Latin America.
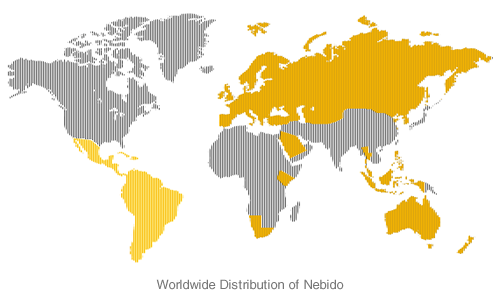
Nebido was licensed by BayerSchering Pharma AG to Indevus Pharmaceuticals, a majority interest in which Endo acquired earlier this year.
Endo Pharmaceuticals is a unit of Endo International; the company renamed itself from Endo Health Solutions February 28 after completing its approximately $2.7 billion cash-and-stock acquisition of Canadian-based Paladin Labs. In addition to Aveed, Endo also markets Delatestryl (T. enanthate) and Fortesta testosterone gel.
Official Endo News Release – DUBLIN, March 6, 2014 – U.S. FDA Approves AVEED™ (Testosterone Undecanoate) Injectable Testosterone Replacement Therapy For Men Living With Hypogonadism, Or Low-T
FDA Approval History for Aveed
There are no long-acting testosterone formulations available in Canada, though testosterone undecanoate is available in Canada in an oral formulation known as Andriol.
Journal Articles
Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals.
Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, Cupisti S, Beckmann MW, Dittrich R.
J Sex Med. 2010 Sep;7(9):3190-8. doi: 10.1111/j.1743-6109.2010.01912.x.
Long-Acting Intramuscular Testosterone Undecanoate for Treatment of Female-to-Male Transgender Individuals
Jens W. Jacobeit MD, Louis J. Gooren MD and Heinrich M. Schulte MD.
Article first published online: 17 JUL 2007. DOI: 10.1111/j.1743-6109.2007.00556.x
Will YOU be looking into Aveed? Leave your comments below!



I am very excited that there is now an FDA-approved form of Nebido in the US. I would absolutely love to switch from my testosterone cypionate to Aveed, but at this point it is unfortunately not an option for me due to the cost (I pay out of pocket, and my insurance likely won’t cover this since it doesn’t cover what I already use).
I hope to make the switch to Aveed one day.
That price tag is ridiculously rough, wow. I’ll stick with the weekly injections.
great that testosterone undeconate finally has found the way to America. But what a fantasy price??!!! In Switzerland we pay 160 Dollar for 1vial. This is unfair. Somebody is very interested to make money out of this.
If my insurance covers it, I will definitely be switching as the bi weekly injection causes me a great deal of pain in my affected muscles and I have extreme needle phobia. One injection every two-three months is a dream!
I would probably use it if it is eventaully avaialbe here in Canada, however I have hesitations. As long as the price comes down or it is covered by insurance. I would want to also see if they could use another oil other than castor oil. The impact of its production can be quite harmful to the workers in he factories that produce it if not done correctly because ricin is a by product of the refining and processing of castor oil. Also, the fact that they are looking at genetic modification of the plant to prevent the byproduct of ricin in the processed of refining the oil is while good for the workers may not be good for patients as reaseaching the long term use of Genetically modified Foods has not really been a priority for a great deal of people, we do not know for sure what thye could be. thought I have the same concern with the Depo T that I was just prescribed as cotton seed oil is already genetical modified in most cases…
I use this kind of T and find it super convenient! It’s great for people who travel because you don’t have to worry about carrying around vials or needles.
I haven’t tried other forms of T so I can’t compare, but I never feel testosterone lows (or peaks) and my blood tests show consistent hormone levels throughout between injections.
I have an appt tomorrow with my endocrinologist and I am hopeful that he is able to prescribe this. I’ve been so excited about this drug.
I am Poland and I was just prescribed Nebido for which i paid here $132 ( 6.6 times less than US) I paid $53 for a visit with the doctor who is the professor of Endocrinology at the Warsaw University. The blood test cost $9.
When it becomes available and affordable, I’ll look into it. I’m glad I got over my dislike of the needles, ans taking one injection every other week is nothing compared to my coworkers and friends who have to take several medications daily, throughout the day!
Aveed is available in the US, but it’s not expected to become affordable.
Here in Costa Rica it costs $76 USD, not sure why the huge price differences,
Roger, is that the price for Aveed specifically in CR? The price difference is because pharmaceutical companies can get away with just about anything in the United States.
Anyway we can request this to available in Canada? Or find out if it’s being looked at by Health Canada?
Good question, CJ. I haven’t seen any indication that Aveed will enter the Canadian marketplace. While it would be advantageous for Canadians to have access to more than just two brands of injectable T, I’m also very wary about how Aveed would be priced in Canada and wouldn’t want to see it disrupt the supply of more affordable brands.
I’ve been using this for over 2 years now in NZ basically for free, and coming back to the US and am realizing how fucked we are. This stuff is so much easier and more level. It’s really infuriating how we basically can’t have this without cutting off an arm…
The reason for the price difference is obvious. The USA is a capitalist society without universal health care. Luckily the USA has enough bombs to kill everything when the people get sick of paying taxes for bombs and not health care. That will solve two problems. The problem of people without health care and the need for health care all together.
In the USA this is so OVERPRICED that insurance companies should refuse to pay for it. Not fair to charge everyone high insurance premiums just because USA politicians and pharmaceutical companies are corrupt and evil. If the insurance companies were to set their payment for drugs to be in line with what other developed countries pay the cost would be so high NO ONE would buy the new drugs and the companies would be forced to lower their rates.
We need to find a place overseas to buy Nebido in bulk. It’s very cheap anywhere but in the US
I feel for all the US guys. I’ve been taking Reandron for 10 years now. It’s a huge 1000mg dose taken every 3 months. Definitely makes everything easier. And it’s covered under (Australian) medicare if your endocrinologist classes you as male with ‘androgen deficiency’. So I only pay $6. I don’t know if this will help anyone internationally…